





Our laboratory is dedicated to advancing the frontiers of immunity, metabolism, and aging. We investigate the molecular mechanisms of immune aging and chronic inflammation, as well as tumor immune evasion and therapeutic target discovery. By integrating metabolomics, single-cell and spatial omics, CRISPR screening, and artificial intelligence, we aim to translate fundamental discoveries into novel strategies for disease intervention and clinical application.

Aging is a key factor contributing to the increase in various morbidity and mortality rates among the elderly population, causing immune decline and chronic inflammation in the immune system, which poses a threat to the health of the elderly. My research focuses on the regulatory mechanisms and potential transformation of metabolism on immune aging. By applying cutting-edge cross-disciplinary technologies such as metabolomics, CRISPR screening, single-cell omics, spatial omics, and artificial intelligence, I investigate the molecular mechanisms of immune decline and chronic inflammation during aging through chemical biology, and develop new intervention plans to reverse aging.
Learn More
We are committed to clarifying the mechanism of the immune system's role in tumorigenesis, especially the molecular basis of tumor immune escape. Through cutting-edge cross-disciplinary technologies such as single-cell omics, spatial omics and artificial intelligence, we seek new anti-tumor immune targets and develop innovative antibody drugs to promote precision immunotherapy.
Learn More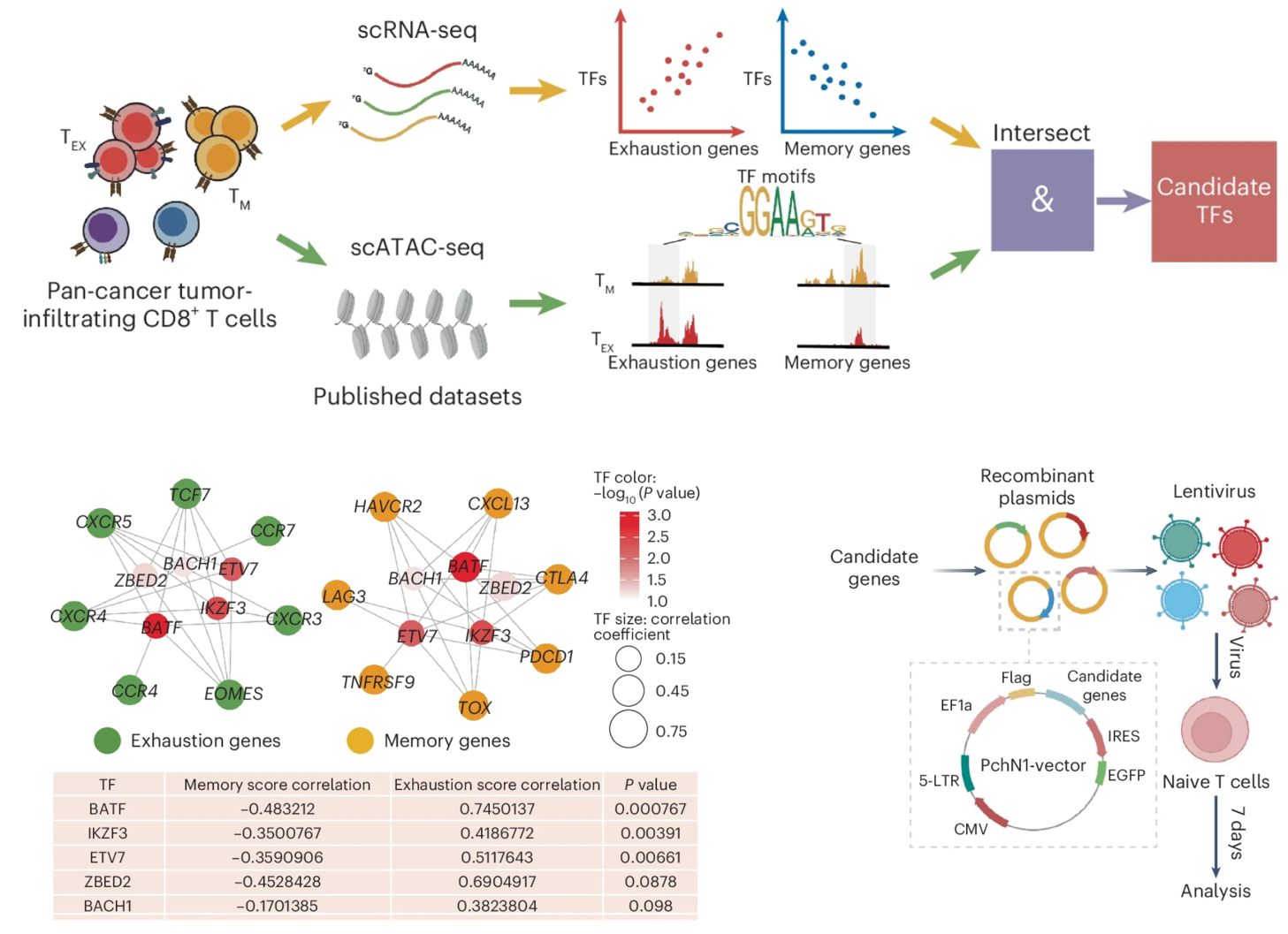
Terminal exhaustion is a critical barrier to antitumor immunity. By integrating and analyzing single-cell RNA-sequencing and single-cell assay for transposase-accessible chromatin with sequencing data, we found that ETS variant 7 (ETV7) is indispensable for determining CD8+ T cell fate in tumors. ETV7 introduction drives T cell differentiation from memory to terminal exhaustion, limiting antiviral and antitumor efficacy in male mice. Mechanistically, ETV7 acts as a central transcriptional node by binding to specific memory genes and exhaustion genes and functionally skewing these transcriptional programs toward exhaustion. Clinically, ETV7 expression is negatively correlated with progression and responsiveness to immune checkpoint blockade in various human cancers. ETV7 depletion strongly enhances the antitumor efficacy of CD8+ T cells and engineered chimeric antigen receptor T cells in solid tumors. Thus, these findings demonstrate a decisive role for ETV7 in driving CD8+ T cell terminal exhaustion and reveal that ETV7 may be a promising target and biomarker for improving the efficacy of cancer immunotherapy.
Learn More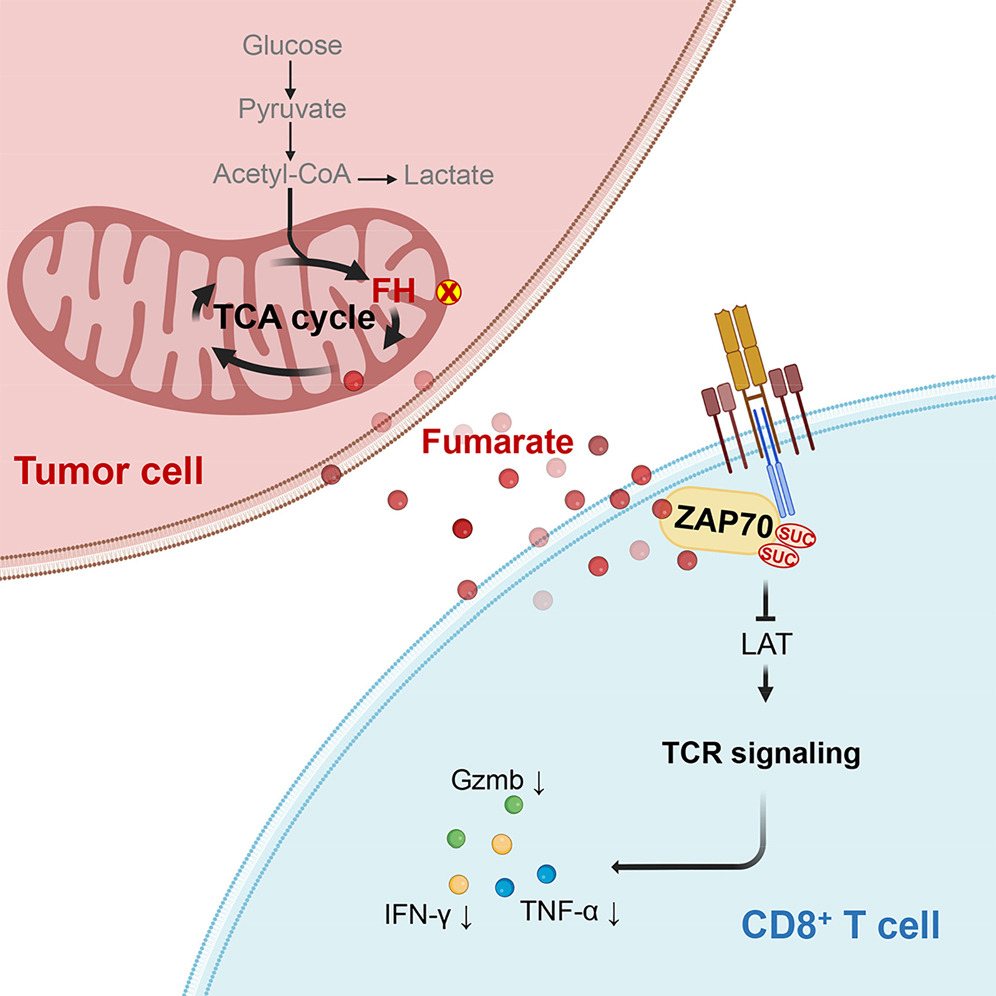
Metabolic alterations in the microenvironment significantly modulate tumor immunosensitivity, but the underlying mechanisms remain obscure. Here, we report that tumors depleted of fumarate hydratase (FH) exhibit inhibition of functional CD8+ T cell activation, expansion, and efficacy, with enhanced malignant proliferative capacity. Mechanistically, FH depletion in tumor cells accumulates fumarate in the tumor interstitial fluid, and increased fumarate can directly succinate ZAP70 at C96 and C102 and abrogate its activity in infiltrating CD8+ T cells, resulting in suppressed CD8+ T cell activation and anti-tumor immune responses in vitro and in vivo. Additionally, fumarate depletion by increasing FH expression strongly enhances the anti-tumor efficacy of anti-CD19 CAR T cells. Thus, these findings demonstrate a role for fumarate in controlling TCR signaling and suggest that fumarate accumulation in the tumor microenvironment (TME) is a metabolic barrier to CD8+ T cell anti-tumor function. And potentially, fumarate depletion could be an important strategy for tumor immunotherapy.Cell metabolism发表专文评述:Fumarate disarms CD8+ T cells against cancer. Jun 6;35(6):907-909.
Learn More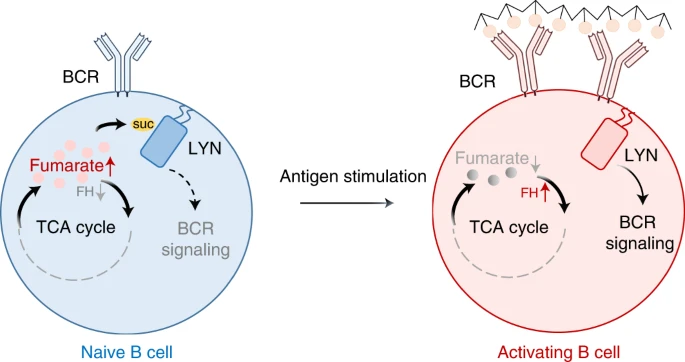
Activated B cells increase central carbon metabolism to fulfill their bioenergetic demands, yet the mechanistic basis for this, as well as metabolic regulation in B cells, remains largely unknown. Here, we demonstrate that B-cell activation reprograms the tricarboxylic acid cycle and boosts the expression of fumarate hydratase (FH), leading to decreased cellular fumarate abun dance. Fumarate accumulation by FH inhibition or dimethyl-fumarate treatment suppresses B-cell activation, proliferation and antibody production. Mechanistically, fumarate is a covalent inhibitor of tyrosine kinase LYN, a key component of the BCR sig naling pathway. Fumarate can directly succinate LYN at C381 and abrogate LYN activity, resulting in a block to B-cell activation and function in vitro and in vivo. Therefore, our findings uncover a previously unappreciated metabolic regulation of B cells, and reveal LYN is a natural sensor of fumarate, connecting cellular metabolism to B-cell antigen receptor signaling.
Learn More

Department of Pathology, School of Basic Medicine, Huazhong University of Science and Technology; Ph.D. supervisor.
Obtained Ph.D. degree from Peking University in 2020. From 2020 to 2024, engaged in postdoctoral research at Tsinghua University. The primary research focus is tumor immunology, with an emphasis on applying metabolomics, CRISPR screening, single-cell omics, and spatial omics technologies to investigate the mechanisms of tumor immune evasion and to identify novel targets for anti-tumor immunity. Another research direction is immunosenescence, particularly exploring the mechanisms of immunosenescence from a metabolic perspective and strategies to reverse it in order to reduce the risk of age-related diseases such as cancer.
Learn More
Doctoral student

Postgraduate

Postgraduate

Postgraduate
Undergraduate

Undergraduate